About CovidSurg Cohort Study
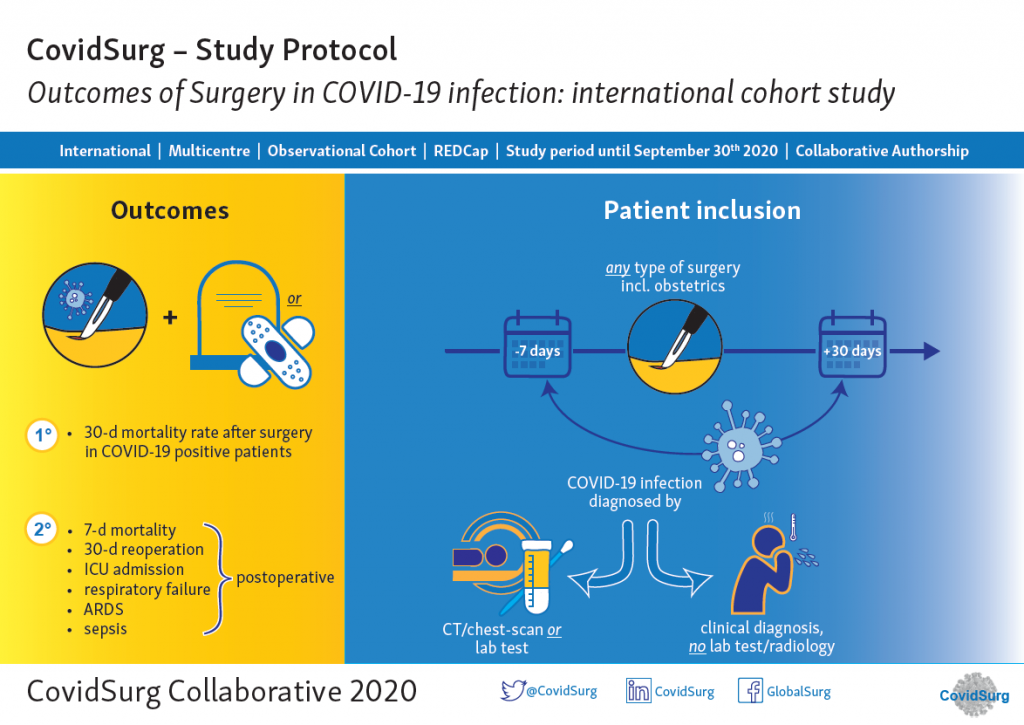
Patients diagnosed with COVID-19 who need surgery are a challenging group. Capturing real-world data and sharing international experience about the management of surgical patients during this pandemic will inform clinical decision making. CovidSurg Cohort Study is an international multi-centric cohort study aiming to assess the outcomes of surgery in patients with SARS-CoV2 infection.
Patients meeting the following inclusion criteria can be included in CovidSurg:
-
Patients undergoing ANY type of surgery in an operating theatre, this includes obstetrics.
AND
-
Either before or after surgery: (i) lab test confirmed SARS-CoV2 infection or (ii) clinical diagnosis of COVID-19 (no test performed).

Highlights of CovidSurg Cohort Study
-
Any hospital can participate. Some hospitals might currently have few cases while others might have a lot. Every hospital treating or expecting to treat surgical patients with COVID-19 infection is welcome.
-
Patients can be included in this study if they undergo any type of surgery and have a COVID-19 diagnosis (by lab test or clinical), in the pre or post-operative period.
-
The primary outcome is mortality at 30 days after surgery. Secondary outcomes will be other surgical and medical complications.
-
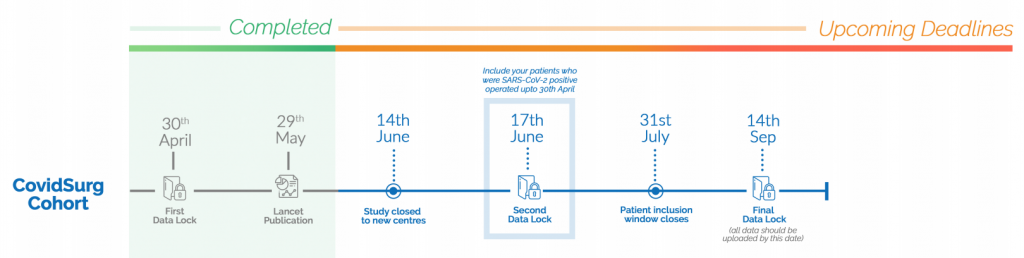
There is no fixed data collection period and patients can be included either prospectively or retrospectively.
-
Data will be stored in a secure online platform (REDCap). Only anonymised data will be collected.
-
All the collaborators will be recognised as co-authors in any publications resulting from this study. A corporate authorship model will be used under CovidSurg Collaborative group, for example: https://pubmed.ncbi.nlm.nih.gov/29452941
Setting up your site to participate in CovidSurg Cohort Study
-
To get involved, please first complete the registration form.
-
Ensuring local approvals to participate is the most important next step.
-
You will get an email from our team and your hospital will be issued a REDCap login. If your hospital is already signed up for the study, you will be out in contact with your colleagues to build a team.
-
If you wish to start data collection on paper and upload it later, please use the data collection sheet below.
-
Contacts for each country are available in our network page so you can have support from your colleagues.
-
For any queries please contact [email protected].
General documents
In order to facilitate communication with patients and local approvals, we have designed:
-
A Patient Information Sheet and an Informed Consent template. You are free to use it or adapt it, according to local requirements.
-
A support/invitation letter from the study coordinator. Some ethics committees may require this. You might also use this as a facilitator to talk about the study to your Head of Department.
-
A letter about the online secure data storage platform – REDCap. Some ethics committees require detailed information about it.
-
An example of a CovidSurg poster designed to be affixed in the operating theatre. The aim is to let all surgical teams know about the study and maximise patient inclusion. It can be used as a template for your own hospital.
-
A link sheet if you wish to have a document cross-referencing the hospital nr and REDCap ID. This can be useful to track patients records to collect follow-up data. You should keep this file in a secure place in your hospital.