The NIHR Global Health Research Unit on Global Surgery
Improving surgical outcomes through collaborative research
CEI & Advocacy
Improving outcomes for patients is central to the work of the GSU. To ensure our research is relevant to communities in LMICs, we involve patients with lived experience and the public in the prioritisation, design and implementation of our clinical trials and studies. This ensures that local healthcare needs are being met and potential barriers and challenges identified and overcome early on, therefore guaranteeing the success of our studies and allowing us to have a greater impact on surgical healthcare in LMICs.

Community Engagement and Involvement (CEI) at GSU
“…the process of working collaboratively with and through groups of people affiliated by geographic proximity, special interest, or similar situations to address issues affecting the well-being of those people”
– US Centers for Disease Control and Prevention
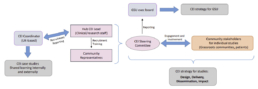
The key building blocks are:
1. Each Hub employs a CEI Lead (e.g. a research nurse), who has been trained in best practice CEI according to the UNICEF standards and is responsible for recruitment, training and ongoing support of the community representatives
2. The community representatives are well-connected key stakeholders with reach into grassroots communities
3. The Hub CEI Lead and community representatives meet regularly to form the (virtual) CEI steering committee
4. The steering committee allows us to reach those in the community who are most affected by our research and ensurse they are empowered to contribute towards decision-making
All CEI activities are being captured and evaluated by the UK-based CEI Coordinator, who is also responsible for training, monitoring and shared learning.

Ghana PPI Community Engagement
GSU Mexico Shares Expertise, Wins Hearts at Mexican Childhood Cancer Association
News,Community Engagement and Involvement,CEI,Mexico,Public Health,SSI
April 23, 2024
In a bid to empower patients, the GSU Mexico Hub held a community engagement event at the DIF Ruiz Cortines Community Centre, Veracruz on wound care and infection prevention on 22nd February.
0 Comments2 Minutes
Mexico Hub Community Event Educates on Wound Care
News,Community Engagement and Involvement,CEI,Education and Training,Mexico,Public Health,SSI
March 8, 2024
In a bid to empower patients, the GSU Mexico Hub held a community engagement event at the DIF Ruiz Cortines Community Centre, Veracruz on wound care and infection prevention on 22nd February.
0 Comments1 Minutes
GSU awarded additional funding for Community Engagement activities
News,Community Engagement and Involvement,CEI,SSI
February 8, 2024
The Global Surgery Unit was recently awarded additional Official Development Assistance (ODA) funding from the University of Birmingham and UK Research and Innovation to increase already existing community engagement activities across its Indian and Rwandan research Hubs.
0 Comments2 Minutes
Launching the GSU Education Centre
February 7, 2024
A community engagement talk organised by the GSU India Hub, attended by 40 paramedic trainees was held at the School for Skills in Allied Health Sciences, Sonarpur, Kolkata, India on October 31, 2023.
0 Comments1 Minutes
Our CEI Case Studies
Our case studies showcase practical application of CEI in LMIC settings with a focus on exploring best practice and highlighting challenges and barriers, as well as potential solutions. Learn about the tailored CEI activities.
THE TIGER STUDY
Task-shifting inguinal hernia repair between surgeons and non-surgeon physicians in rural Ghana.
THE STARFISH STUDY
Stoma Care For Improvement Research: Epidemiologic study of stoma cases in The Philippines and qualitative research on the challenges of stoma care.
Bringing together surgeons, researchers and policy makers to set the local research agenda according to patient need in LMIC and ensuring all patients have the opportunity to take part in our research.




















