CovidSurg-Cancer closing stages
Many thanks to all collaborators who have contributed to the COVIDSurg-Cancer project to date. Patient identification has now ended in all centres and we are launching for the closing stages of the project.
We are aware that tracking non-operated patients was not possible in all centres, due to capacity or the design of your local care pathways.
As such, we now need to identify a team classification (group 1 or group 2) for your specialty in your hospital to help us keep track of follow-up of patients within the study. Please read the below carefully to identify your team classification and what to do next.
![]()
Watch this short video with a discussion between two teams.
Group 1
-
Select Group 1 if your team can accurately include all eligible operated and non-operated patients
-
Teams in group 1 will be included in analyses (and authorship) for papers both describing outcomes of operated and non-operated patients
-
Please complete this form to indicate your team classification as Group 1
-
Please identify and enter all eligible non-operated or delayed patient records onto REDCap as soon as possible
-
Please ensure that you keep a careful record of all patients linked local Medical Record numbers and REDCap IDs (infographic here)
-
Follow-up data: this can be entered in the existing ‘Management’ form on REDCap
-
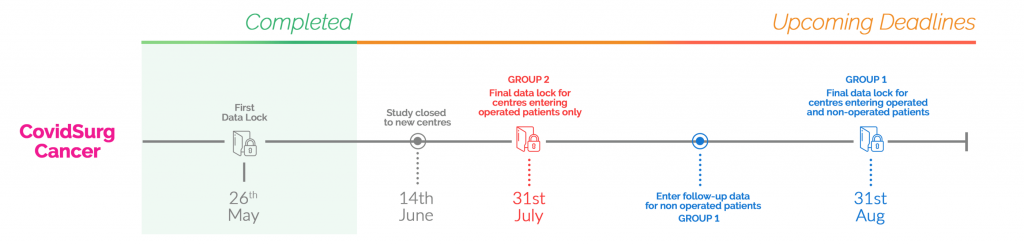
We are aiming to end follow-up for these patients on 31st August 2020 and want as up to date data as possible to be entered regarding the status of these patients by this date.
-
For some patients this may be a shorter length of follow-up (e.g. those identified in early June will have under 3 months follow-up), for some this will represent a longer length of follow-up(e.g. those identified in March will have almost 6-months follow-up).
-
Please enter the final date of follow-up of each non-operated patient’s records (ideally as close to 31st August 2020 as possible) and confirm inclusion of non-operated patients.
-
-
If the patient has an operation before 31st August 2020, the ‘clock stops’ and 30-day postoperative outcome data should be collected in the same way as any other operated patient.
-
The following infographic helps to explain the follow-up pathway for specialties/centres in Group 1.
To print this checklist, please click here.
Group 2
-
Select Group 2 if your team can accurately include eligible operated patients ONLY
-
Teams in group 2 will be included only in analyses (and authorship) for papers describing outcomes of operated patients
-
Please complete this form and indicate your team classification as Group 2
-
Please ensure that all consecutive eligible patients have been included within your patient identification window
-
You can include operated patients until 31st July 2020 – patients entered after this date will not be included in analyses
-
Please ensure that all relevant datapoints including 30-day follow-up is complete for all operated patients
To print this checklist, please click here.
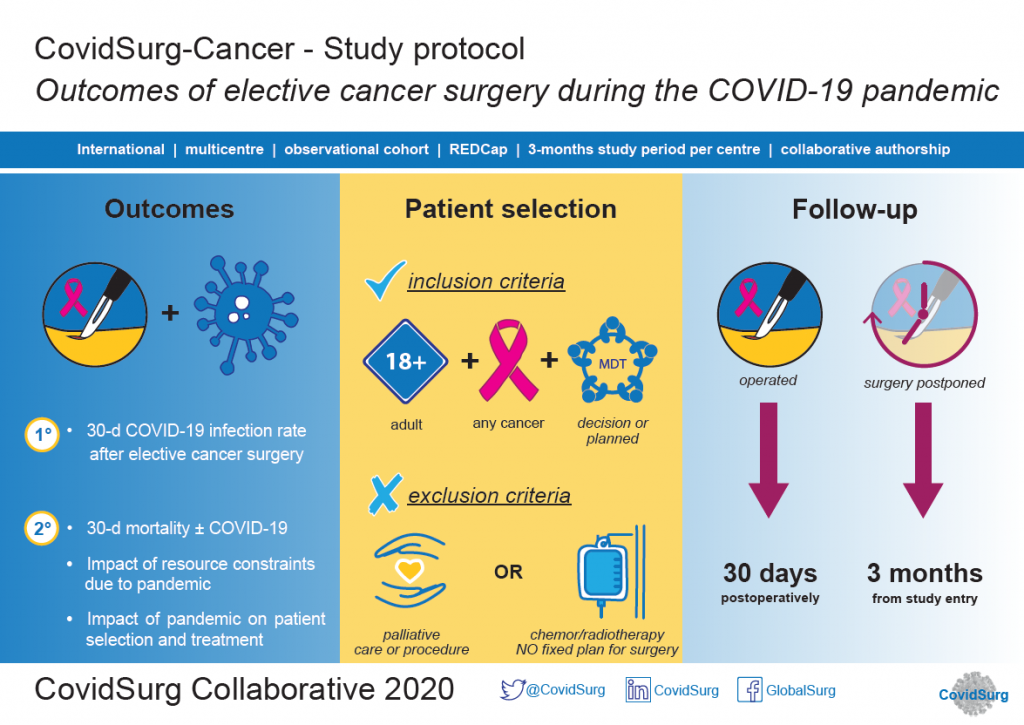
About CovidSurg-Cancer
The rapid emergence of the SARS-CoV2 virus across international borders has forced the redesign of clinical services to deprioritise non-urgent surgical care. Hospitals and health systems need to continue managing cancer patients, for whom a delay in treatment can lead to poorer outcomes. On the other side, maintaining treatment for cancer can put cancer patients at higher risk of post-operative complications due to the current pandemic.
There is an emergent need to better understand the impact of COVID-19 on care of cancer patients requiring surgery.

Highlights of CovidSurg-Cancer
Hospitals:
-
Any hospital performing elective cancer surgery can participate.
-
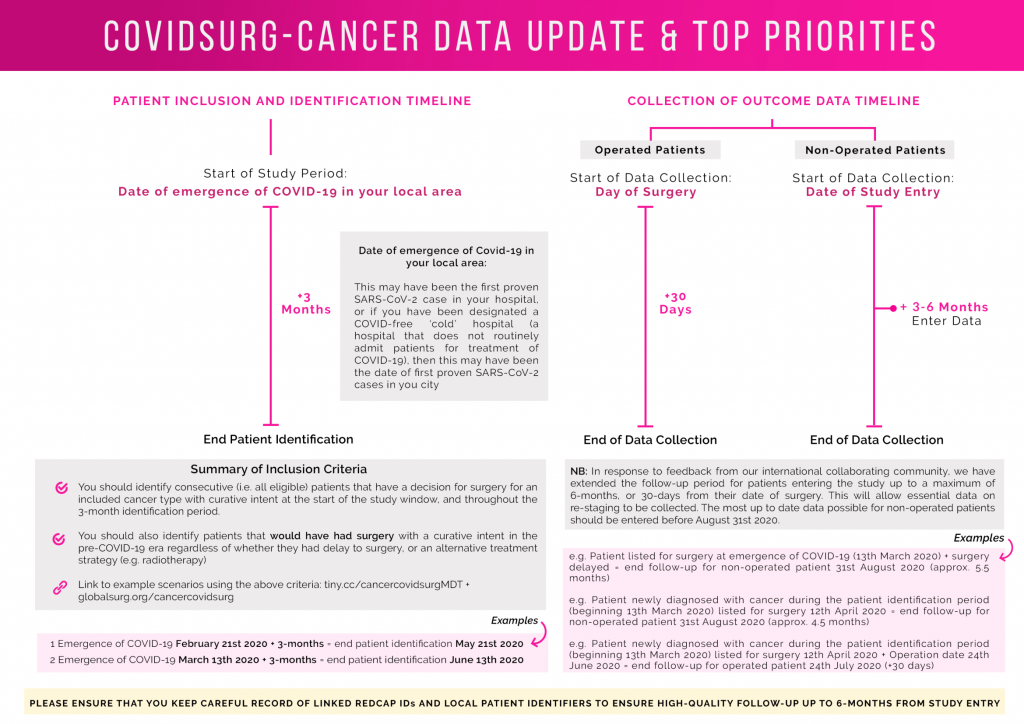
Investigators can enrol patients with confirmed diagnoses of colorectal, oesophagogastric, head and neck, lung, hepatopancreataobiliary, urological, neurological, gynaecological, breast cancers and soft-tissue or bone sarcoma. As a rapid response study to the COVID-19 pandemic, included cancer types have evolved following a short pilot period.
Patient inclusion criteria:
-
Patients planned for curative cancer surgery that have surgery completed during the COVID-19 pandemic.
-
Patients that would have been planned for curative surgery in the pre-COVID-era that have surgery delayed or cancelled during the COVID-19 pandemic.
Outcomes:
-
Primary outcome: 30-day postoperative COVID-19 infection rate;
-
Secondary outcomes: 30-day postoperative mortality and critical care admission; delay in surgery > 4 weeks; progression to incurable disease at 3 months.
Data collection period: Three months since the starting date (identified by local investigators as the emergence of COVID-19 in their hospital).
Data storage: Data will be stored in a stored in a secure online platform (REDCap). Only anonymised data will be collected.
Authorship: All the collaborators will be recognised as co-authors in any publications resulting from this study. A corporate authorship model will be used under CovidSurg Collaborative group, for example: https://pubmed.ncbi.nlm.nih.gov/29452941
Local approvals for CovidSurg-Cancer
Obtaining local approval is the most important step after registering your interest. Please find below the study protocol that you can use for that.
The global community has recognised that rapid dissemination and completion of studies in COVID-19 infected patients is a high priority. Please discuss with your head of department to identify the quickest way to secure study approval. A data collection sheet is available if you would like to collect data whilst awaiting formal study approval.
In order to facilitate communication with patients and local approvals, we have designed:
-
A Patient Information Sheet and Informed Consent template. You are free to use it or adapt it, according to local requirements.
-
A letter from the Royal College of Surgeons supporting CovidSurg.
-
An example of a CovidSurg-Cancer local approval.
-
A letter about the online secure data storage platform – REDCap. Some ethics committees may require detailed information about it.
-
A poster you can use to disseminate information about the study and help tracking patients.
Country specific documents
Specialty hub
[contact-form-7 id=”17330″ title=”CovidSurg_specialty_hub”]