September NewsletterThe Global Surgical Outcomes Collaboration |
|
|
|
GlobalSurg 3: the story so far
|
|
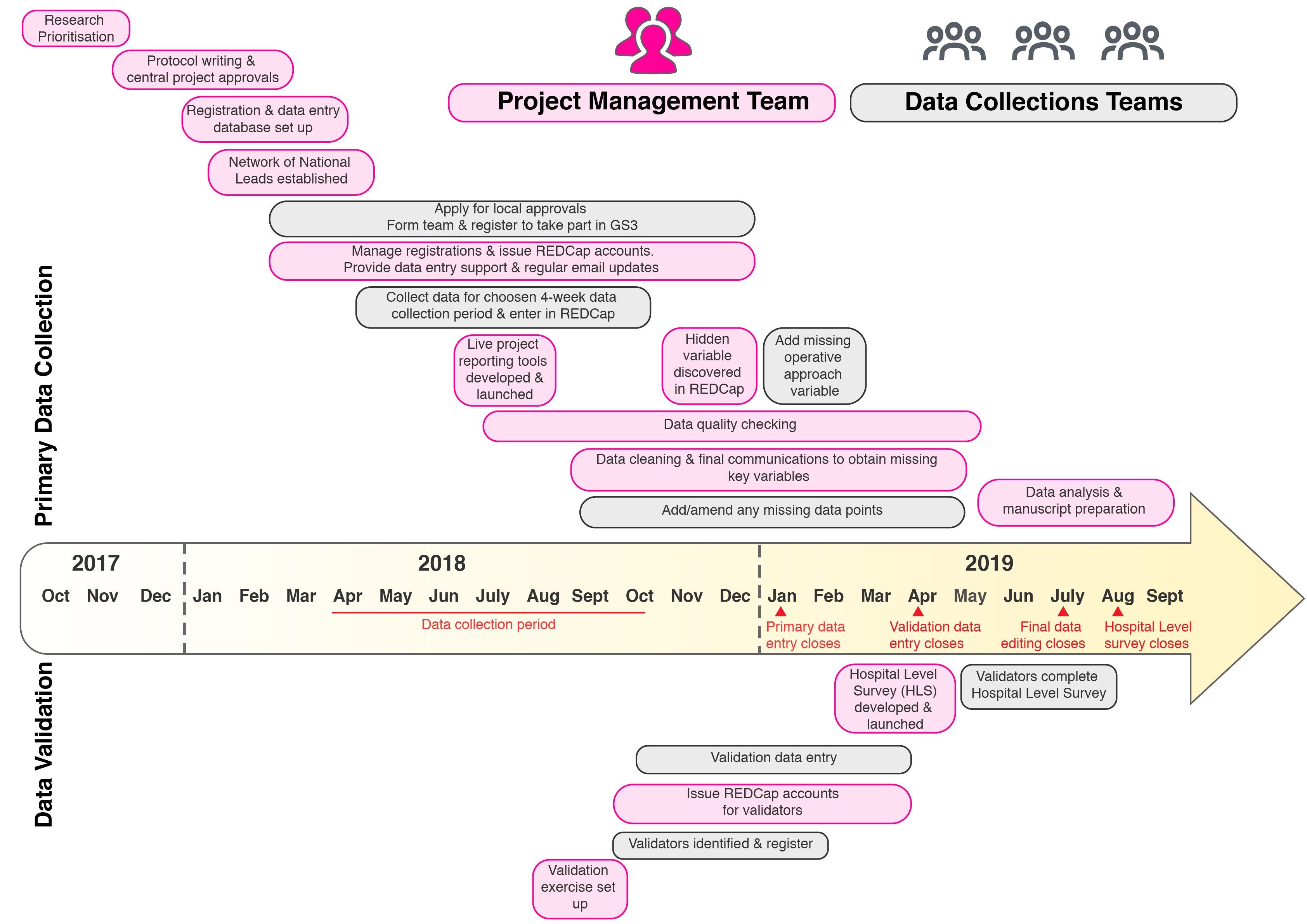
GlobalSurg 3 is now is the final stages of data analysis and manuscript preparation. It has been our biggest and most complex study to date and has relied on the hard work and dedication of literally thousands of individuals all around the world. The GlobalSurg team have recently been reflecting on quite how much has been achieved in a relatively short period of time – from initial study inception in Johannesburg back in November 2017, to a fully validated dataset of over 16,000 surgical cancer patients less than 2 years later. Not to mention the establishment of a network of >100 National Leads, the development of live data reporting tools, new means of communication with our teams (Podcasts, SlackChats, blogs) and the beginning of the SurgStreet collaboration with the GapMinder Foundation that have happened along the way. We realise that for teams who joined the study at right at the beginning, it may seem there has been a lag between their contribution of 4-weeks of data in April 2018 and the generation of the results and publications. However, there has been a lot going on in this time! We have put together the figure above to illustrate just how the study has been progressing and how the project management team and data collection teams have been interacting to generate the final, fully validation GlobalSurg 3 dataset. It been a busy few years for everyone taking part in GlobalSurg 3 but we have learned a lot and established cutting edge tools and techniques in the field of medical data collection on a truly global scale – we hope you’ve enjoyed it as much as we have! |
|
|
| Early Results
The data is still being fully analysed, but you can see the preliminary patient inclusion numbers below – also available on the GlobalSurg Data Centre – follow data.globalsurg.org/numbers for updates.
|
|
|
| What Happens Next?
The next major task for the project management team, along side the data analysis and manuscript writing, is production of the study authorship list. Our inclusive publication policy means that every individual who actively contributed data to the study will be named as a collaborator on all publications arising from the study. We are now in the process of generating the Authorship for the GlobalSurg 3 publications. It is a massive undertaking to make sure everyone who contributed data to the study is included. The final list will contain >2000 individuals in >80 countries. Use of the ORCID ID will make this process much more straight forward than has been the case previously. We will shortly circulate a PDF containing the names, listed by country and hospital, of all those who will be included in the final authorship list. In the meantime, you can help by ensuring your name is correct on your ORCID ID profile, and is exactly as you wish it to appear on the final publication – we will not be accepting any corrections to name spellings by email; you must make changes on your ORCID ID profile. Only accidental omission or inclusions will be dealt with by email. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|









Leave A Comment